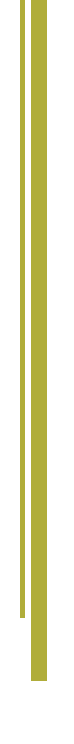Structural Heart
Structural heart defects vary in intensity and level of seriousness. Some defects are natural from birth and are no cause for concern. Others may require immediate care due to discomfort or the possibility of greater damage to your overall health. A variety of treatment options are available for atrial septal defect, patent foramen ovale, and valvular heart disease.
Learn more about specific heart conditions and treatments below.
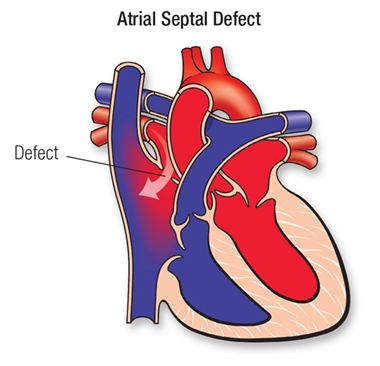
Atrial Septal Defect
Atrial Septal Defect is a “hole” in the wall that separates the top two chambers of the heart.
This defect allows oxygen-rich blood to leak into the oxygen-poor blood chambers in the heart. ASD is a defect in the septum between the heart’s two upper chambers (atria). The septum is a wall that separates the heart’s left and right sides.
MORE INFORMATION FOR PARENTS OF CHILDREN WITH ASD
Every child is born with an opening between the upper heart chambers. It’s a normal fetal opening that allows blood to detour away from the lungs before birth. After birth, the opening is no longer needed and usually closes or becomes very small within several weeks or months.
Sometimes the opening is larger than normal and doesn’t close after birth. In most children the cause isn’t known. Some children can have other heart defects along with ASD.
Normally, the left side of the heart only pumps blood to the body, and the right side of the heart only pumps blood to the lungs. In a child with ASD, blood can travel across the hole from the left upper heart chamber (left atrium) to the right upper chamber (right atrium) and out into the lung arteries. If the ASD is large, the extra blood being pumped into the lung arteries makes the heart and lungs work harder, and the lung arteries can become gradually damaged. If the hole is small, it may not cause symptoms or problems. Many healthy adults still have a small leftover opening in the wall between the atria, sometimes called a Patent Foramen Ovale (PFO).
Children with an ASD often have no symptoms. If the opening is small, it won’t cause symptoms because the heart and lungs don’t have to work harder. If the opening is large, the only abnormal finding may be a murmur (noise heard with a stethoscope) and other abnormal heart sounds. In children with a large ASD, the main risk is to the blood vessels in the lungs because more blood than normal is being pumped there. Over time, usually many years, this may cause permanent damage to the lung blood vessels.
 If the opening is small, it doesn’t make the heart and lungs work harder. Surgery and other treatments may not be needed. Small ASDs that are discovered in infants often close or narrow on their own. There isn’t any medicine that will make the ASD get smaller or close any faster than it might do naturally.
If the opening is small, it doesn’t make the heart and lungs work harder. Surgery and other treatments may not be needed. Small ASDs that are discovered in infants often close or narrow on their own. There isn’t any medicine that will make the ASD get smaller or close any faster than it might do naturally.
If the ASD is large, it can be closed with open-heart surgery, or by cardiac catheterization using a device inserted into the opening to plug it. Sometimes, if the ASD is an unusual position within the heart, or if there are other heart defects such as abnormal connections of the veins bringing blood from the lungs back to the heart (pulmonary veins), the ASD can’t be closed with the catheter technique. In that case, surgery is needed.
Closing a large ASD by open-heart surgery usually is done in early childhood, even in patients with few symptoms, to prevent complications later. Many defects can be sewn closed without using a patch.
MORE INFORMATION FOR ADULTS WITH ASD
The cause is usually unknown. Genetic factors can sometimes play a role.
If the hole is small, it may have minimal effect on heart function. When a large defect exists between the atria, a large amount of oxygen-rich (red) blood leaks from the heart’s left side back to the right side. Then this blood is pumped back to the lungs, despite already having been refreshed with oxygen. Unfortunately, this creates more work for the right side of the heart.
This extra amount of blood flow in the lung arteries can also cause gradual damage.
Some patients with ASD have no symptoms. If the opening is small, it won’t cause symptoms because the additional work done by the heart and lungs is minimal. If the opening is large, it may cause mild shortness of breath, especially with exercise. The increased blood in the lung may increase a patient’s susceptibility to pneumonia and bronchitis.
On physical examination, the only abnormal finding may be a murmur (noise heard with a stethoscope) and other abnormal heart sounds. However, with progressive damage to the lung vessels, the pressures in the lung may rise, and the patient can become more severely limited, eventually developing Eisenmenger’s syndrome, described below.
A large ASD is usually closed in early childhood, even in patients with few symptoms, to prevent complications later. Some defects were closed with a patch of pericardium (the normal lining outside the heart) or synthetic material such as Dacron. However, many defects that required surgery may have been sewn closed without using a patch. The prognosis after ASD closure during childhood is excellent, and late complications are uncommon.
 If the opening is small, surgery or other treatments may not be needed.
If the opening is small, surgery or other treatments may not be needed.
Most large atrial septal defects now can be closed either with open-heart surgery or during a cardiac catheterization using a device inserted into the opening to plug it.
However, if the ASD is in an unusual position within the heart, or if there are other heart defects such as abnormal connections of the veins bringing blood from the lungs back to the heart (pulmonary veins), the ASD cannot be closed with the catheter technique. Then surgery is needed. Even when the defect is discovered in adulthood, patients benefit from closure of large defects.
People with small unrepaired or repaired atrial septal defects rarely have any late problems. Those who have palpitations or who faint need to be evaluated by their cardiologist and may need medical therapy.
Also, if the ASD is diagnosed late in life, the heart’s ability to pump may have been affected, leading to heart failure. This condition can require diuretics, drugs to help the heart pump better and drugs to control blood pressure. If pulmonary hypertension develops (which is uncommon), some people may need extra medications.
Patients who have had a transient ischemic attack (TIA) or a stroke and are found to have a PFO may be treated with aspirin or another blood thinner. If another stroke recurs on medicines, patients may be referred to have a PFO or small ASD closed.
There are now special studies in progress to determine whether medications or closure of the PFO is better at preventing stroke. It is important to emphasize that the vast majority of people with small PFOs and ASDs don’t have strokes and don’t need to have their defects closed.
ONGOING CARE
Patients with a history of ASD should be seen periodically by a cardiologist to look for uncommon problems. For a short time after surgery to close an ASD, a cardiologist must regularly examine you. The long-term outlook is excellent, and usually no medicines and no additional surgery or catheterization are needed.
Sometimes medicines to prevent blood clots and infection are used for a few months after ASD closure. Only rarely will patients need to take medicine after six months. Your cardiologist can monitor you with noninvasive tests if needed. These include electrocardiograms, Holter monitors, exercise stress tests, and echocardiograms. They will help show if more procedures, such as a cardiac catheterization, are needed.
Most patients with small, unrepaired atrial septal defects and repaired ASDs do not need any special precautions and may be able to participate in normal activities without increased risk.
After recent surgery or catheter closure, your cardiologist may advise some limits on your physical activity for a short time, even when there is no pulmonary hypertension. After successful healing from surgery or catheter closure, no restrictions are usually needed.
The exception is that patients who have developed high pressures in the lungs (pulmonary hypertension – see Eisenmenger’s syndrome) should refrain from high-level sports.
This isn’t needed beyond six months after repair either by surgery or device.
Once the ASD is closed and there’s no leftover opening, the risk with pregnancy is very low. The risk from a pregnancy goes up if there’s an unrepaired ASD, but pregnancy is usually safe unless there is pulmonary hypertension.
A large unrepaired ASD may sometimes lead to heart failure during pregnancy, but this is usually well controlled with medication if caught early. There is a slight risk of stroke during pregnancy, so precautions against blood clots may be recommended.
Once an ASD has been closed, it’s unlikely that more surgery will be needed. Rarely, a patient may have a residual hole. Whether it will need to be closed depends on its size.
Patent Foramen Ovale (PFO)
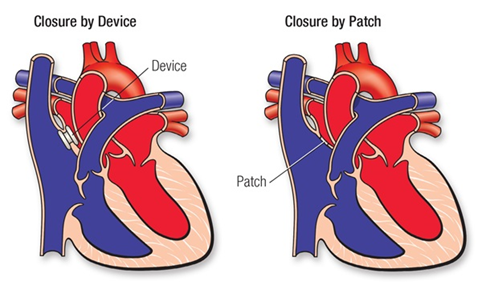
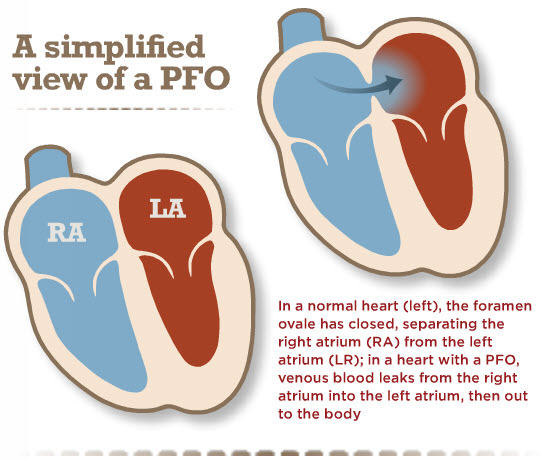 A hole in your heart would seem to be the very definition of a “problem.” Yet more than a quarter of the population has one, and for most it causes no adverse health effects. In fact, the vast majority of those affected don’t even know it.
A hole in your heart would seem to be the very definition of a “problem.” Yet more than a quarter of the population has one, and for most it causes no adverse health effects. In fact, the vast majority of those affected don’t even know it.
There are two kinds of holes in the heart. One is called an atrial septal defect (ASD), and the other is a patent foramen ovale (PFO). Although both are holes in the wall of tissue (septum) between the left and right upper chambers of the heart (atria), their causes are quite different.
An ASD is a failure of the septal tissue to form between the atria, and as such it is considered a congenital heart defect, something that you are born with. Generally an ASD hole is larger than that of a PFO. The larger the hole, the more likely there are to be symptoms.
PFOs, on the other hand, can only occur after birth when the foramen ovale fails to close. The foramen ovale is a hole in the wall between the left and right atria of every human fetus. This hole allows blood to bypass the fetal lungs, which cannot work until they are exposed to air. When a newborn enters the world and takes its first breath, the foramen ovale closes, and within a few months it has sealed completely in about 75 percent of us.
When it remains open, it is called a patent foramen ovale, patent meaning open. For the vast majority of the millions of people with a PFO, it is not a problem, even though blood is leaking from the right atrium to the left. Problems can arise when that blood contains a blood clot.
Finding out whether you have a PFO is not easy, and it’s something that isn’t usually investigated unless a patient is having symptoms like severe migraines, TIA, or stroke. Although the prevalence of PFO is about 25 percent in the general population, this increases to about 40 to 50 percent in patients who have stroke of unknown cause, referred to as cryptogenic stroke. This is especially true in patients who have had a stroke before age 55. In some cases, the PFO combines with another condition, such as atrial fibrillation, to increase the risk of stroke.
Transcatheter Aortic Valve Replacement (TAVR/TAVI)
This minimally invasive surgical procedure repairs the valve without removing the old, damaged valve. Instead, it wedges a replacement valve into the aortic valve’s place. The surgery may be called a transcatheter aortic valve replacement (TAVR) or transcatheter aortic valve implantation (TAVI).
Somewhat similar to a stent placed in an artery, the TAVR approach delivers a fully collapsible replacement valve to the valve site through a catheter. Once the new valve is expanded, it pushes the old valve leaflets out of the way and the tissue in the replacement valve takes over the job of regulating blood flow.
This procedure is fairly new and is FDA approved for people with symptomatic aortic stenosis who are considered an intermediate or high risk patient for standard valve replacement surgery. The differences in the two procedures are significant.
Usually valve replacement requires an open-heart procedure with a “sternotomy,” in which the chest is surgically separated (open) for the procedure. The TAVR or TAVI procedures can be done through very small openings that leave all the chest bones in place.
A TAVR procedure is not without risks, but it provides beneficial treatment options to people who may not have been candidates for them a few years ago while also providing the added bonus of a faster recovery in most cases. A patient’s experience with a TAVR procedure may be comparable to a balloon treatment or even an angiogram in terms of down time and recovery and will likely require a shorter hospital stay (average 3-5 days).
The TAVR procedure is performed using one of two different approaches, allowing the cardiologist or surgeon to choose which one provides the best and safest way to access the valve:
- Entering through the femoral artery (large artery in the groin), called the transfemoral approach, which does not require a surgical incision in the chest, or
- Using a minimally invasive surgical approach with a small incision in the chest and entering through a large artery in the chest or through the tip of the left ventricle (the apex), which is known as the transapical approach.
At this time the procedure is reserved for those people for whom an open-heart procedure poses intermediate risk. For that reason, most people who have this procedure are in their 70s or 80 and often have other medical conditions that make them a better candidate for this type of surgery.
TAVR can be an effective option to improve quality of life in patients who otherwise have limited choices for repair of their aortic valve.